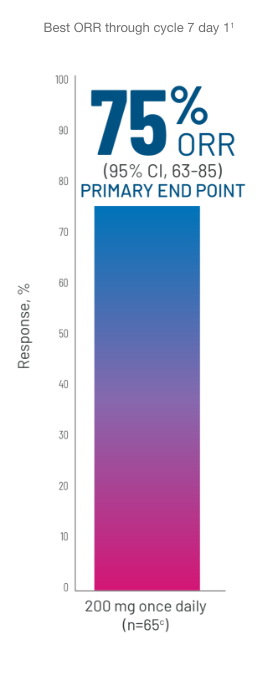
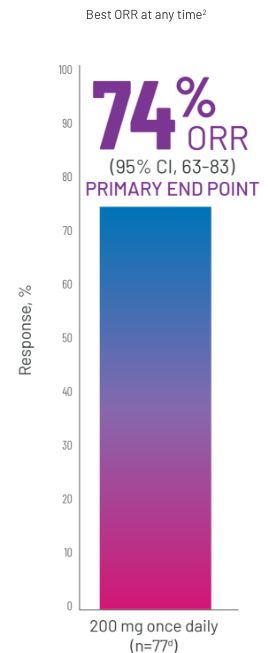
ORRb FOLLOWING TREATMENT WITH REZUROCK 200 mg ONCE DAILY
-

CR, n=4 (6%). PR, n=45 (69%).1
aLong-term follow-up period is defined as 3 years.3
bBest ORR was defined as the proportion of patients who achieved CR or PR according to the 2014 NIH cGVHD Consensus Criteria.4
cBased on a final analysis by the FDA as seen through cycle 7 day 1 (n=65).1
dBased on mITT population (n=77).2
-

CR, n=4 (5%). PR, n=53 (69%).3
aLong-term follow-up period is defined as 3 years.3
bBest ORR was defined as the proportion of patients who achieved CR or PR according to the 2014 NIH cGVHD Consensus Criteria.4
cBased on a final analysis by the FDA as seen through cycle 7 day 1 (n=65).1
dBased on mITT population (n=77).2
Long-term analysisa study limitations
This is an open-label follow-upa analysis of the mlTT population.2,3 Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn. Clinical data were analyzed based on all available data, including the potential continued involvement of responders and attrition of nonresponders. All patients received REZUROCK. Therefore, patients served as their own baseline control. This analysis includes data from an additional 20 patients who were enrolled in a subsequent biomarker cohort after the August 19, 2020, ROCKstar publication data cutoff, and therefore comprises 152 patients, including the addition of 11 patients in the once-daily arm and 9 patients in the twice-daily arm. The addition of these patients may impact the data due to their limited time on therapy.2-4 aLong-term follow-up period is defined as 3 years.3
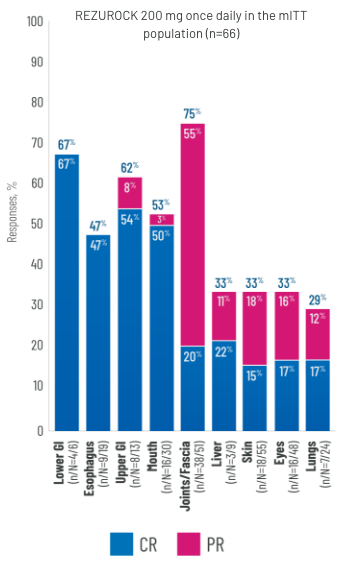
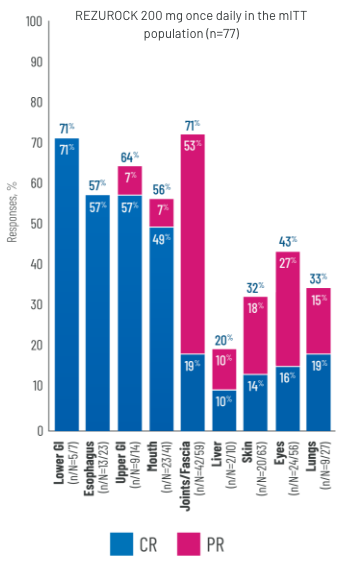
RESPONSES BY ORGAN SYSTEM
-

aLong-term follow-up period is defined as 3 years.3
ePercentages may not add up to the total due to rounding.
-

Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
aLong-term follow-up period is defined as 3 years.3
ePercentages may not add up to the total due to rounding.
MEDIAN TTNTf,g WITH REZUROCK 200 mg ONCE DAILY IN THE mITT POPULATION
-

aLong-term follow-up period is defined as 3 years.3
fTTNT was defined as the time from the first dose of REZUROCK to the start of additional systemic cGVHD therapy.3
gPrespecified secondary end point; not powered to show statistical significance.
-

Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
aLong-term follow-up period is defined as 3 years.3
fTTNT was defined as the time from the first dose of REZUROCK to the start of additional systemic cGVHD therapy.3
gPrespecified secondary end point; not powered to show statistical significance.
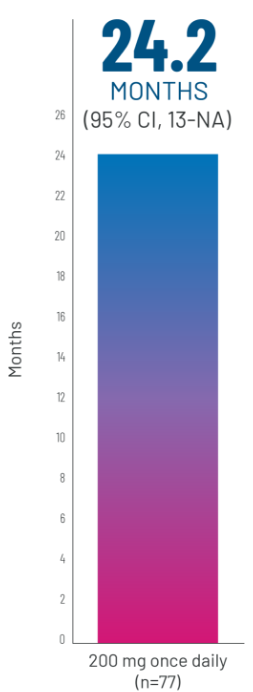
FFSh WITH REZUROCK 200 mg ONCE DAILY IN THE mITT POPULATION
-

Kaplan-Meier curve of estimated FFS.3aLong-term follow-up period is defined as 3 years.3
gPrespecified secondary end point; not powered to show statistical significance.
hFFS was defined as the absence of relapse, nonrelapse mortality or a need for additional systemic therapy.4
-

Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
Kaplan-Meier curve of estimated FFS.3aLong-term follow-up period is defined as 3 years.3
gPrespecified secondary end point; not powered to show statistical significance.
hFFS was defined as the absence of relapse, nonrelapse mortality or a need for additional systemic therapy.4
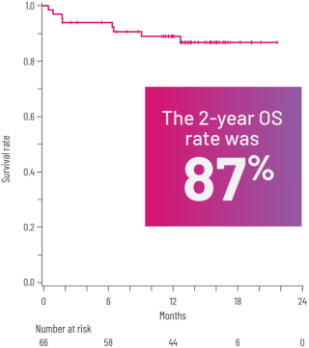
OSi WITH REZUROCK 200 mg ONCE DAILY IN THE mITT POPULATION
-

Kaplan-Meier curve of estimated OS.3
aLong-term follow-up period is defined as 3 years.3
gPrespecified secondary end point; not powered to show statistical significance.
iOS was defined as the time from the first dose of REZUROCK to the date of death due to any cause.3
-

Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
In the long-term follow-up analysis, 3-year OS data were not available. The 2-year OS includes 11 additional patients from a subsequent biomarker cohort after the August 19, 2020, ROCKstar publication data cutoff.3Kaplan-Meier curve of estimated OS.3
aLong-term follow-up period is defined as 3 years.3
gPrespecified secondary end point; not powered to show statistical significance.
iOS was defined as the time from the first dose of REZUROCK to the date of death due to any cause.3
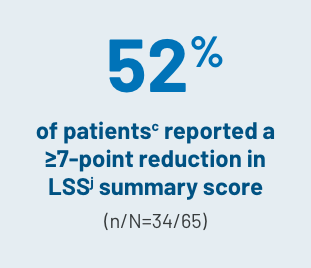
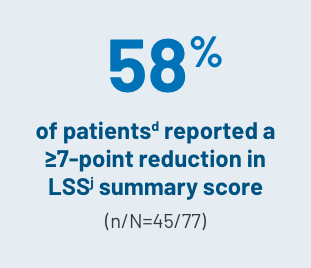
CHANGE IN QOL SCORES
-
REZUROCK 200 mg ONCE DAILY IN AN
EXPLORATORY ANALYSIS
aLong-term follow-up period is defined as 3 years.3
cBased on a final analysis by the FDA as seen through cycle 7 day 1 (n=65).1
dBased on the mITT population (n=77).2
jThe LSS is a 30-item, 7-subscale symptom scale and QOL measurement tool that evaluates the AEs of cGVHD in the categories of skin, vitality, lung, nutritional status, psychological functioning, eye and mouth.5
-
REZUROCK 200 mg ONCE DAILY IN AN
EXPLORATORY ANALYSIS
Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
aLong-term follow-up period is defined as 3 years.3
cBased on a final analysis by the FDA as seen through cycle 7 day 1 (n=65).1
dBased on the mITT population (n=77).2
jThe LSS is a 30-item, 7-subscale symptom scale and QOL measurement tool that evaluates the AEs of cGVHD in the categories of skin, vitality, lung, nutritional status, psychological functioning, eye and mouth.5
CHANGE IN CS AND CNI DOSE IN THE 200-mg ONCE-DAILY ARM
-
Patients who received CS therapy4


Patients who received CNI therapy3


aLong-term follow-up period is defined as 3 years.3
fBased on mITT population (n=77).2
mBased on mITT population (n=66).4
aLong-term follow-up period is defined as 3 years.3
dBased on mITT population (n=77).2
kBased on mITT population (n=66).4 -
Patients who received CS therapy3


Patients who received CNI therapy3


Data presented are descriptive in nature. No conclusions of statistical significance or clinical significance can be drawn.
aLong-term follow-up period is defined as 3 years.3
dBased on mITT population (n=77).2
kBased on mITT population (n=66).4
LEARN ABOUT THE SAFETY PROFILE FOR REZUROCK.
AE, adverse event; cGVHD, chronic graft-versus-host disease; CNI, calcineurin inhibitor; CR, complete response; CS, corticosteroid; FDA, US Food and Drug Administration; FFS, failure-free survival; GI, gastrointestinal; LSS, Lee Symptom Scale; mITT, modified intent-to-treat; MOA, mechanism of action; NA, not available; NIH, National Institutes of Health; ORR, overall response rate; OS, overall survival; PR, partial response; QOL, quality of life; TTNT, time to next treatment.
References: 1. REZUROCK. Package insert. Kadmon Pharmaceuticals, LLC. 2. Lee SJ, Cutler C, Pavletic S, Blazar BR, Yao Y, Ji R; on behalf of the ROCKstar Study Investigators. Belumosudil for chronic graft-versus-host disease after 2 or more lines of systemic therapy: 3-year follow-up to the ROCKstar study. Poster presented at: Tandem Meetings; February 21-24, 2024; San Antonio, TX. 3. Data on file. Sanofi. 4. Cutler C, Lee SJ, Arai S, et al; on behalf of the ROCKstar Study Investigators. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138(22):2278-2289. doi:10.1182/blood.2021012021 5. Lee SJ, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(8):444-452. doi:10.1053/bbmt.2002.v8.pm12234170
INDICATION
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.



.png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 400w, /.imaging/webp/sanofi-platform/img-w500/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 500w, /.imaging/webp/sanofi-platform/img-w600/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 600w, /.imaging/webp/sanofi-platform/img-w700/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 700w, /.imaging/webp/sanofi-platform/img-w800/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 800w, /.imaging/webp/sanofi-platform/img-w900/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 900w, /.imaging/webp/sanofi-platform/img-w1200/dam/rezurock-hcp/long-term-analysis-data/m-2.3_FFS-with-REZUROCK_Long-Term_Line_42_RGB_PV2-(1).png/jcr:content/m-2.3_FFS%20with%20REZUROCK_Long%20Term_Line_42_RGB_PV2%20(1).png 1200w)
.png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 400w, /.imaging/webp/sanofi-platform/img-w500/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 500w, /.imaging/webp/sanofi-platform/img-w600/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 600w, /.imaging/webp/sanofi-platform/img-w700/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 700w, /.imaging/webp/sanofi-platform/img-w800/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 800w, /.imaging/webp/sanofi-platform/img-w900/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 900w, /.imaging/webp/sanofi-platform/img-w1200/dam/rezurock-hcp/long-term-analysis-data/m-2.3_OS-Long-Term_Line_86_RGB_PV1-(2).png/jcr:content/m-2.3_OS%20Long%20Term_Line_86_RGB_PV1%20(2).png 1200w)