The inflammatory and fibrotic manifestations of cGVHD have widespread clinical impacts1
Patients who undergo an alloHCT can develop complications, such as debilitating and life-threatening cGVHD.2-4 In fact, cGVHD is the leading cause of nonrelapse mortality in patients surviving >2 years post alloHCT.3
Risk factors for identifying patients who are more likely to develop cGVHD5-8
- Prior aGVHD
- No ATG use
- Older age
- Bone marrow source
- RIC
- HLA mismatch
- Female donor to male recipient
- Peripheral blood stem cell source
- High numbers of infused T cells
- Positive CMV serology
Multiple organs can be affected by cGVHD9
ORGANS AFFECTED BY cGVHD AT THE TIME OF DIAGNOSIS

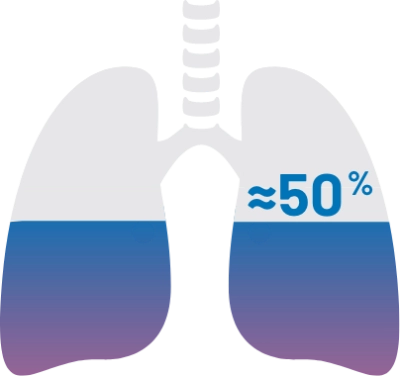
According to a study of a prospectively assembled cohort of patients with cGVHD, approximately half of patients had lung manifestations.10
Treatment of fibrosis in the lungs is a critical unmet need.11-14
Inflammation and fibrosis
The relationship between inflammation and fibrosis in cGVHD is complex and not fully understood.15,16 However, there is an association between chronic inflammatory changes in cGVHD and collagen-producing fibroblasts.17,18 This can lead to the development of fibrotic lesions across multiple organs.17,19
- Significant morbidity and life-threatening complications are largely the result of fibrosis,20 which can affect extensive areas of the skin21 and involve other organs, such as the lungs16,19
- Although some aspects of inflammation in cGVHD can be addressed, there is limited evidence regarding the effectiveness of current treatments on fibrosis22-28
See how REZUROCK works
Voice-over: The mechanism of action of REZUROCK (belumosudil).
REZUROCK (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Please see additional Important Safety Information presented at the end of this video.
REZUROCK is an innovative therapy designed for the treatment of chronic graft-versus-host disease, or chronic GVHD. As a selective ROCK2 inhibitor, REZUROCK simultaneously targets both the inflammatory and fibrotic components of chronic GVHD.1,2 REZUROCK has a 100-fold higher inhibitory activity against ROCK2 over the ROCK1 isoform.1
In the chronic inflammatory phase of chronic GVHD, uncontrolled activation of Th17 and follicular helper T cells leads to extensive production of pro-inflammatory cytokines, such as IL-17 and IL-21, and B-cell–derived pathologic antibodies.3
Within the naive T cell, ROCK2 interacts with and phosphorylates STAT3, leading to formation of the JAK2/STAT3 complex and upregulation of Th17 and follicular helper T cells.1,4,5
REZUROCK interrupts this process through its selective inhibition of ROCK2.1 This results in the decreased activation of STAT3, triggering the significant downregulation of both Th17 and follicular helper T cells.1,4
In addition, injury to the thymus from conditioning regimens used prior to transplant, immunosuppressant-based prophylaxis post transplant and other factors contributes to its inability to produce a normal amount of regulatory T cells.3 As a result, autoreactive and alloreactive T cells escape regulation and contribute to lasting chronic inflammation in chronic GVHD.3,6
REZUROCK promotes the interaction of ROCK2 with JAK3, which increases phosphorylation of STAT5, causing upregulation of regulatory T cells.5
The immunomodulatory effect of REZUROCK on STAT3 and STAT5 phosphorylation reduces the inflammation associated with chronic GVHD.1,4
In addition to the chronic inflammatory state that continues through this fibrotic phase of the pathophysiology of chronic GVHD, activated macrophages of the innate immune system produce growth factors that activate profibrotic mediators, such as LPA and TGF-β.7,8 These mediators subsequently activate ROCK2,7,8 which results in polymerization of G-actin to F-actin.7 This frees the transcription factor MRTF and leads to transcription of profibrotic genes.7 The process leads to changes to the cellular structure and an increase in tissue stiffness, which are the key features of fibrotic diseases.7
Selective ROCK2 inhibition with REZUROCK prevents the polymerization of G-actin to F-actin, as well as MRTF-induced upregulation of profibrotic gene expression.7
This results in the ability of REZUROCK to downregulate fibrosis, as evidenced by the decreased collagen deposition around the bronchioles and the delayed progression of scleroderma in animal chronic GVHD models.2
In summary, REZUROCK restores immune homeostasis in chronic GVHD by downregulating Th17 cells and upregulating regulatory T cells.1,5 Simultaneously, REZUROCK downregulates the fibrotic processes of chronic GVHD.2 As an oral selective ROCK2 inhibitor, REZUROCK is an innovative therapy designed for the treatment of chronic GVHD.1,2
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
-
The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
-
Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
-
Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
-
Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
-
Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
-
Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
-
Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
-
Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
-
Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
-
Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
-
Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
-
Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
-
Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
aGVHD, acute graft-versus-host disease; alloHCT, allogeneic hematopoietic cell transplant; ATG, antithymocyte globulin; cGVHD, chronic graft-versus-host disease; CMV, cytomegalovirus; GI, gastrointestinal; HLA, human leukocyte antigen; MOA, mechanism of action; RIC, reduced-intensity conditioning.
References: 1. Flowers MED, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125(4):606-615. doi:10.1182/blood-2014-08-551994 2. Arora M, Pidala J, Cutler CS, et al. Impact of prior acute GVHD on chronic GVHD outcomes: a Chronic Graft-versus-Host Disease Consortium study. Leukemia. 2013:27(5):1196-1201. doi:10.1038/leu.2012.292 3. Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239. doi:10.1200/JCO.2010.33.7212 4. Arai S, Arora M, Wang T, et al; for the Graft-vs-Host Disease Working Committee of the CIBMTR. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266-274. doi:10.1016/j.bbmt.2014.10.021 5. Afram G, Pérez Simón JA, Remberger M, et al. Reduced intensity conditioning increases risk of severe cGVHD: identification of risk factors for cGVHD in a multicenter setting. Med Oncol. 2018;35(6):79. doi:10.1007/s12032-018-1127-2 6. Chen Y-B, Wang T, Hemmer MT, et al. GvHD after umbilical cord blood transplantation for acute leukemia: an analysis of risk factors and effect on outcomes. Bone Marrow Transplant. 2017;52(3):400-408. doi:10.1038/bmt.2016.265 7. Lazaryan A, Weisdorf DJ, DeFor T, et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched sibling donors. Biol Blood Marrow Transplant. 2016;22(1):134-140. doi:10.1016/j.bbmt.2015.09.008 8. Kok LMC, Bungener L, de Bock GH, et al. Risk factors associated with the development of moderate to severe chronic graft-versus-host disease after non-myeloablative conditioning allogeneic stem cell transplantation in patients with AML or MDS. Hum Cell. 2020;33(1):243-251. doi:10.1007/s13577-019-00297-7 9. Data on file. Kadmon Pharmaceuticals, LLC; 2018. 10. Jacobsohn DA, Kurland BF, Pidala J, et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120(13):2545-2552. doi:10.1182/blood-2012-04-424135 11. Arora M, Cutler CS, Jagasia MH, et al. Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3):449-455. doi:10.1016/j.bbmt.2015.10.018 12. Inagaki J, Moritake H, Nishikawa T, et al. Long-term morbidity and mortality in children with chronic graft-versus-host disease classified by National Institutes of Health Consensus Criteria after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(11):1973-1980. doi:10.1016/j.bbmt.2015.07.025 13. Inamoto Y, Martin PJ, Chai X, et al; on behalf of the Chronic GVHD Consortium. Clinical benefit of response in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(10):1517-1524. doi:10.1016/j.bbmt.2012.05.016 14. Gazourian L, Spring L, Meserve E, et al. Pulmonary clinicopathological correlation after allogeneic hematopoietic stem cell transplantation: an autopsy series. Biol Blood Marrow Transplant. 2017;23(10):1767-1772. doi:10.1016/j.bbmt.2017.06.009 15. Kitko CL, White ES, Baird K. Fibrotic and sclerotic manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1 suppl):S46-S52. doi:10.1016/j.bbmt.2011.10.021 16. Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(2):211-234. doi:10.1016/j.bbmt.2016.09.023 17. MacDonald KPA, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1)13-21. doi:10.1182/blood-2016-06-686618 18. Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565-2579. doi:10.1056/NEJMra1703472 19. Fiuza-Luces C, Simpson RJ, Ramírez M, Lucia A, Berger NA. Physical function and quality of life in patients with chronic GvHD: a summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transplant. 2016;51(1):13-26. doi:10.1038/bmt.2015.195 20. Henden AS, Hill GR. Cytokines in graft-versus-host disease. J Immunol. 2015;194(10):4604-4612. doi:10.4049/jimmunol.1500117 21. Chronic graft-vs-host disease of skin and connective tissues. BMT InfoNet. Accessed June 2, 2023. Bmtinfonet.org/video/chronic-graft-vs-host-disease-skin-and-connective-tissues 22. Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129(1):22-29. doi:10.1182/blood-2016-08-686659 23. Jakafi. Package insert. Incyte Corporation; 2023. 24. Modi B, Hernandez-Henderson M, Yang D, et al. Ruxolitinib as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2019;25(2):265-269. doi:10.1016/j.bbmt.2018.09.003 25. Imbruvica. Package insert. Pharmacyclics LLC; 2022. 26. Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9(1):21-46. doi:10.1177/2040620717741860 27. Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130-137. doi:10.1182/blood-2016-02-702852 28. REZUROCK. Package Insert. Kadmon Pharmaceuticals, LLC. 29. Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci USA. 2014;111(47):16814-16819. doi:10.1073/pnas.1414189111 30. Flynn R, Paz K, Du J, et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood. 2016;127(17):2144-2154. doi:10.1182/blood-2015-10-678706
INDICATION
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for one week after the last dose
Adverse Reactions
- The most common (≥ 20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension
- Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in > 3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥ 2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each)
- Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly
Drug Interactions
- Strong CYP3A Inducers: Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers
- Proton Pump Inhibitors: Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure, which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors
- Certain UGT1A1 substrates: Avoid coadministration of REZUROCK with UGT1A1 substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the UGT1A1 substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of UGT1A1. Coadministration of REZUROCK with a UGT1A1 substrate decreased plasma concentrations of the glucuronide metabolite, which may increase the risk of adverse reactions related to sensitive substrates of UGT1A1
- Certain P-gp, OATP1B1, and BCRP substrates: Avoid coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp, OATP1B1, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information. REZUROCK is an inhibitor of P-gp, OATP1B1, and BCRP. Coadministration of REZUROCK with P-gp, OATP1B1, and BCRP substrates increased their plasma concentrations, which may increase the risk of adverse reactions related to these substrates
Use in Specific Populations
- Pregnancy: There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus
- Lactation: There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for one week after the last dose
- Pediatric Use: The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established
- Geriatric Use: Of the 186 patients with chronic GVHD in clinical studies of REZUROCK, 26% were 65 years and older. No clinically meaningful differences in safety or effectiveness of REZUROCK were observed in comparison to younger patients
- Renal Impairment: Treatment with REZUROCK has not been studied in patients with pre-existing severe renal impairment. For patients with pre-existing severe renal impairment, consider the risks and potential benefits before initiating treatment with REZUROCK
- Hepatic Impairment: Avoid use in patients with moderate hepatic impairment (Child-Pugh B) or severe hepatic impairment (Child-Pugh C) without liver GVHD. No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A)
Please click here for full Prescribing Information.
INDICATION
REZUROCK® (belumosudil) is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
